What kind of microscopes are Serenity and Morpheus?
Both are Zeiss Lightsheet Z.1 instruments used for selective plane illumination microscopy (SPIM). More on SPIM below.
Why the names Serenity and Morpheus?
All MIC instruments have traditionally been nicknamed with sci-fi references in mind. Serenity is named after the space craft in the TV series Firefly (and spin-off movie, Serenity).

In early 2015, the MIC acquired 3 new instruments as part of the Zeiss/BRAIN-MIC initiative. Since there were 3 instruments, we named them Trinity, Neo, and Morpheus, after the main characters in The Matrix Trilogy:

What’s SPIM?
SPIM, selective plane illumination microscopy, is a form of light sheet fluorescence microscopy (LSFM). In contrast to traditional light microscopy, where the illumination and detection are done through the same objective lens, SPIM utilizes 2 objective lenses that are perpendicular to each other. The illumination optics include a cylindrical lens that shapes the laser beam into a thin plane or “sheet” of light. This sheet of light illuminates an entire plane within the sample, allowing image creation to be done with a CCD camera instead of a point detector like on a laser scanning confocal microscope. Thus, confocal-like imaging can be done at camera-speeds, i.e., in excess of 30 frames per second.
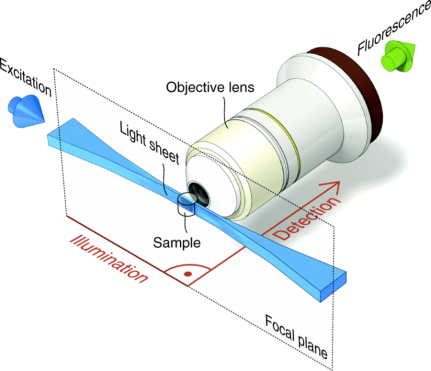

What’s the difference between Serenity and Morpheus?
Both are Zeiss Lightsheet Z.1’s, but Serenity is optimized for water-based samples or objects in a 1.33 refractive index media, such as water, while Morpheus can be used for water-based samples and for cleared samples with a refractive index of 1.45. This is due to additional optics in the lightpath optimized for the differences in refractive indices. So Morpheus is great for samples cleared with CLARITY, CUBIC, FocusClear, SeeDB, etc.
What objectives do Serenity and Morpheus have?
Serenity has 3 objectives for imaging samples in water: 5X/0.16 (air), 20X/1.0 W, and 40X/1.0 W.
Morpheus also has 3 objectives: two for imaging cleared (1.45 refractive index): 5X/0.16 (air) and 20X/1.0 CLARITY; and one for imaging samples in water: 20X/1.0 W.
What kind of detectors are on Serenity and Morpheus?
Each instrument is equipped with two pco.edge sCMOS cameras.
What dyes can I image on Serenity and Morpheus?
Serenity is equipped with 4 excitation lasers: 405, 488, 561, and 635 nm for imaging fluorophores such as DAPI, GFP, RFP, and Cy5, or similar.
Morpheus has 6 laser lines: 405, 440, 488, 514, 561, and 642 nm for imaging DAPI, CFP, GFP, YFP, RFP, and Cy5, or similar.
If you plan to image more than one dye, please check that your dyes will work with the emission filter set combinations.
How does sample mounting work?
Unlike a traditional light microscope where samples are mounted onto a stage, lightsheet samples are immersed into a chamber filled with liquid. The most common mounting methods so far have been embedding live samples in low melting point agarose in a glass capillary or syringe or afixing tissue to a magnet or securing with a custom holder. If you plan to use the Lightsheet, we encourage you to discuss various mounting options with us before you start your experiments/staining.
 Fish in capillary
Fish in capillary
 Brain on hanger
Brain on hanger
 Brain going into chamber
Brain going into chamber
 Setup in a Zeiss lightsheet microscope
Setup in a Zeiss lightsheet microscope
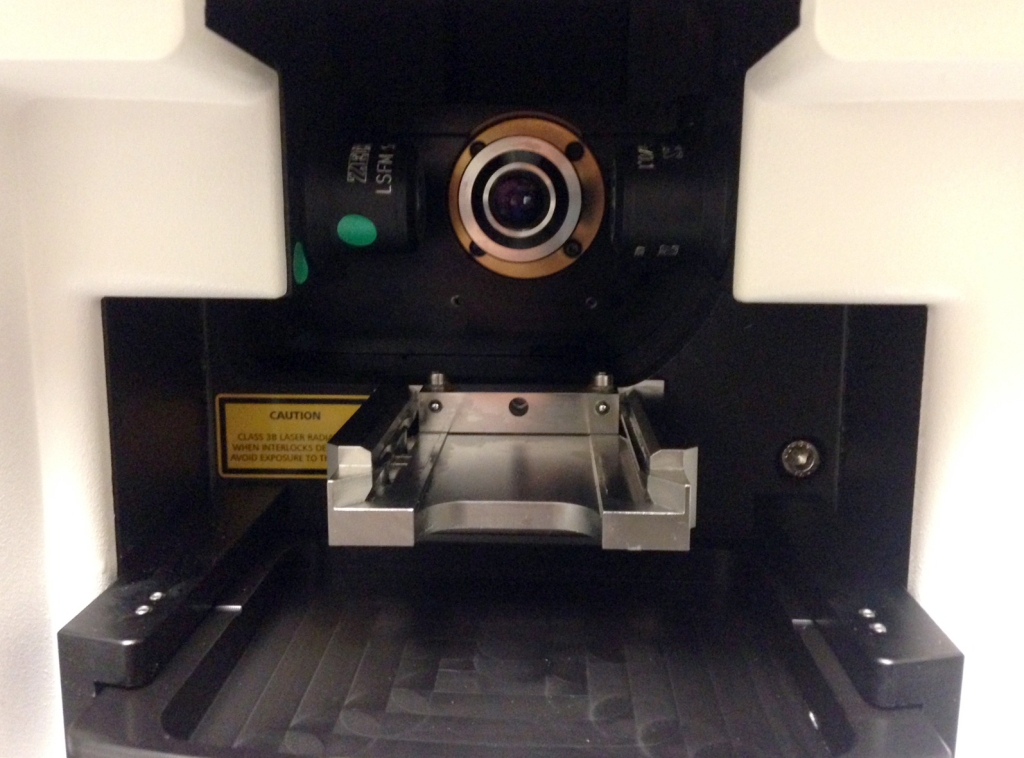 Peeking inside the Zeiss lightsheet microscope. The two illumination objectives are one either side of the detection objective, which is seen head-on.
Peeking inside the Zeiss lightsheet microscope. The two illumination objectives are one either side of the detection objective, which is seen head-on.
 The imaging chambers for the Lightsheet
The imaging chambers for the Lightsheet
 An imaging chamber inside the Lightsheet. Samples are lowered into the imaging chamber from above.
An imaging chamber inside the Lightsheet. Samples are lowered into the imaging chamber from above.
What kind of projects are most suitable for Serenity and Morpheus?
The main reason most people want to use the Lightsheet is to obtain fast, high resolution, 3D (and 4D) images with very low phototoxicity/photobleaching. Serenity has been used to look at development and structures in intact Drosophila egg chambers and wing discs, zebrafish neurons, and mouse retina. Morpheus users have imaged a range of cleared tissues and organs, including brain (whole and lobes/thick sections), pancreas, lung, and liver
Video of a sea monkey imaged on Serenity
Video of a cleared mouse brain imaged on Morpheus
What can I expect from a typical imaging session?
The main differences between imaging on a Lightsheet compared to a standard confocal are the unique mounting setup (see above) and the data processing post-acquisition. Once the imaging parameters are set and the lightsheets are focused, the actual acquisition time is very fast. For data processing, there’s a separate computer at each instrument dedicated to that task. For analysis and presentation, we recommend Arivis or Imaris, both of which are available to MIC users. File sizes can be quite large, so an external hard drive (4 TB or larger) is highly recommended.